Topics around tima-oculav®
Background
Especially in case of accidents with corrosive substances (acids or alkalis), immediate flushing - e.g. with plenty of water - is the first choice. This general recommendation has been confirmed by practical experience in companies and has been incorporated into the rules and regulations of the German Social Accident Insurance Institution and statutory obligations.
Immediate flushing of the eyes, skin or mucous membranes is crucial to dilute and remove e.g. corrosive or toxic substances as quickly as possible so that damage can be kept to a minimum.
Body and eye showers must be accessible for immediate use within 10 seconds or less.
If no flowing drinking water is available nearby, or if a drinking water pipeline would have to be laid at disproportionate expense, eye wash bottles containing sterile rinsing fluid offer a safe alternative.
When and how is tima-oculav® used?
tima-oculav® eye wash is a medical device flushing the eyes, the conjunctival sac, and the skin.
To be used after chemical burns caused by:
- Acids or alkalis
- Pepper spray
- CS combat gas
- Tear gas
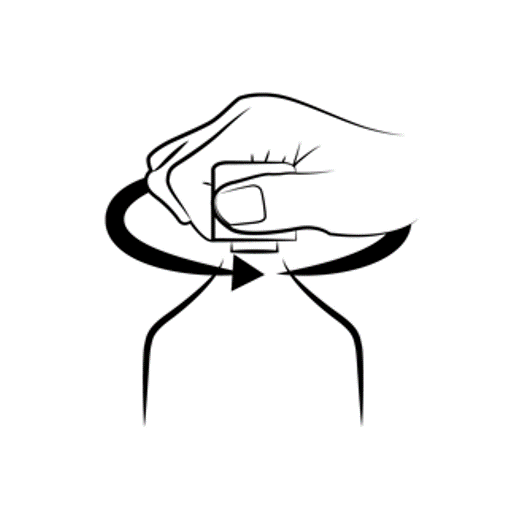
Twist off the cap

Carefully open the eyelids
with your fingers due to the
natural eyelid closure reflex
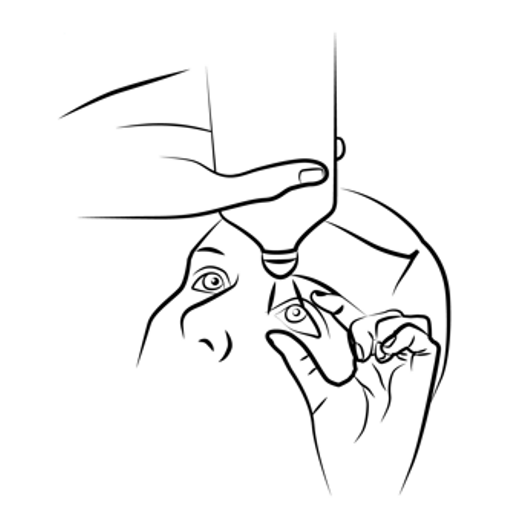
Rinse the affected
area carefully
To ensure the sterility of the bottle's contents, tima-oculav® is intended for single use only.
What are chemical burns?
Chemical burns are tissue destruction caused by the action of acids or alkalis. The corrosives (acids and alkalis) are substances that destroy cell protein and thus lead to extensive necrosis, i.e. local tissue death in a living organism.
A chemical burn with an acid leads to coagulation necrosis, i.e. tissue death as a result of protein coagulation.
A chemical burn with an alkali leads to colliquative necrosis, i.e. tissue death with liquefaction of the damaged tissue. The alkali reacts with the fats in the tissue and forms soaps, which then lead to rapid and deep penetration into the tissue.
The corrosive properties of alkali are caused by the hydroxide ions (OH- - ions), while the hydrogen ions (H+ - ions) cause the corrosive properties of acids. The degree of tissue damage caused by an acid or alkali is therefore primarily dependent on the concentration of free H+ or OH- ions.
In order to keep the tissue damage as low as possible in the case of a chemical burn, it is necessary to bind the H+ ions of the acid or OH- ions of the alkali. The corrosives diffuse very quickly into the interior of the eye, for example alkalis in less than 2 minutes.
Attention:
All corrosive substances trigger an eyelid spasm on first contact with the eye, which can initially only be overcome by carefully opening the eye with spread thumbs and index fingers. Otherwise, the eyelid spasm can only be broken by local anaesthetics, which, however, are firstly not available at the place of the accident and secondly would delay the urgently needed immediate flushing of the eye.
For this reason, the use of Eye Wash bottles with a so-called "eye bathtub" may be critical, because the person affected, or the assistant is prevented from opening the eyelid by the top piece.
In addition, the fluid exchange in the "bathtub" may be insufficient, which even delays and complicates the necessary wash out of the corrosive substance.
Why phosphate buffer for chemical burns?
The effect of the phosphate buffer is based on the interception reaction of hydrogen ions (H+) of the acid or hydroxide ions (OH-) of the alkali.
This means that the acid or alkali is neutralised by the buffer substance, as the H+ ions of the acid or the OH- ions of the alkali combine with the zwitterions of the phosphate buffer.
The phosphate buffer solution as a flushing liquid offers the great advantage over simple flushing with water that, due to the binding of the H+ or OH- ions by chemical reaction, significantly less liquid is required for neutralisation.
For example, to neutralise the amount of only one microlitre of a 1-molar sodium hydroxide solution, 107 µl, i.e. 10 litres of water, are required.
However, such a quantity is often not available at the place of the accident. Moreover, it is technically impossible to bring this amount of liquid to the eye within a few minutes.
In this case, about 25 ml of the phosphate buffer solution is sufficient to achieve the identical neutralisation effect.
In addition, the phosphate buffer solution offers the great advantage that with thorough flushing even the deeper layers of the tissue can be reached and the free H+ or OH- ions that have already penetrated can be bound, which cannot be achieved with rinsing with water.
First aid for lime burns
Lime burns are particularly dangerous because the (neutral) tissue water becomes alkaline through the reaction and further damages the eye.
In lime burns, the protein in the tissue liquefies and combines with lime to form complexes. This cauterising scab is soft and continues to cauterise if it is not removed (so-called colliquative necrosis).
Attention:
Quicklime (= burnt lime, Calcium oxide, CaO) must not be flushed before the lime particles have been mechanically removed from the eye, because otherwise the lime in the eye will be react, releasing thermal energy and aggravating the burn:
Quicklime reacts with water to form calcium hydroxide (hydrated lime):
CaO + H2O ⇌ Ca(OH)2
This reaction is strongly exothermic, i.e. heat is released. During reaction temperatures over 100° C can occur, creating thermal injuries.